-Stage 1.2-
Minerals: Advanced
This page is dedicated to some of the more interesting things about minerals, as well providing a greater understanding about them. Minerals are mostly known for the way gemstones look or how minerals crystals can develop but there are many minerals found in your everyday life that you don't notice.
Previous Step
Next Step
Mineralogy Help
When I was in mineralogy (in the year 2000), learning over 100 minerals as well as their chemical formulas and properties was probably the hardest thing in geology I have ever done. So I made a study sheet that hopefully could help some of you. Just click on the box to download it.
Chemical Formula
Minerals are sorted according to their chemical formula.
The formula has 2 parts; the cation and the anion.
Cation - The first half of the chemical formula. It contains a positive charge is the mineral were to be dissolved.
Anion - The second half of the chemical formula. It contains a negative charge and what is used to categorized minerals.
Example #1 - HCl - Halite (AKA salt)
H - Cation
Cl - Anion
- Grouped as Chloride (due to the Chlorine anion)
Example #2 - KAlSi3O8 - Orthoclase
KAl - Cation
Si3O8 - Anion
- A silicate due to Si and O group
Most anions are more than one element (like Si and O) that form a tight bond that cannot be easily broken
Mineral Structure
The properties of a specific mineral always remain the same because the structure of a mineral is always the same. Sometimes there are two or more minerals with the same chemical formula, however different mineral structures produce minerals with different properties.
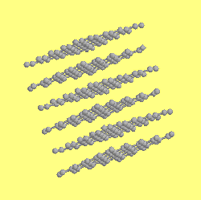
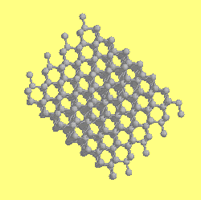
Graphite and Diamond both have Carbon (C) as their chemical formula
Since graphite is formed into sheets, it falls apart much more easily than diamond which is formed into an impenetrable lattice
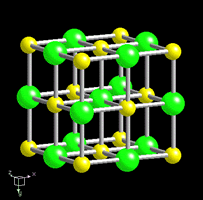
Many mineral properties, like cleavage, are also a result of the crystal structure
The square shape of the structure causes the mineral to break off in tiny cubes (a perfect example of cleavage in 3 directions @ 90°)
Abundance
-
Most minerals are not that common. This is because they are either formed in extreme circumstances, are destroyed easily, or the building blocks for those specific minerals are just not present.
-
Some special minerals, usually the ones with the most basic structure and elements, are extremely common. These can even be found often in your own backyard. These include: